|
hc8meifmdc|20005939267D|healthm_live|health_library|health_library_details|0xfdff91bd010000006e01000001000100
| Overview |
Glutathione is involved in many processes in the body, including tissue building and repair, making chemicals and proteins needed in the body, and for the immune system. Glutathione is a substance produced naturally by the liver.
People take glutathione by mouth for treating cataracts and glaucoma, preventing aging, treating or preventing alcoholism, asthma, cancer, heart disease (atherosclerosis and high cholesterol), hepatitis, liver disease, diseases that weaken the body’s defense system (including AIDS and chronic fatigue syndrome), memory loss, Alzheimer’s disease, osteoarthritis, and Parkinson’s disease. Glutathione is also used for maintaining the body’s defense system (immune system) and fighting metal and drug poisoning. Glutathione is breathed in (inhaled) for treating lung diseases, including idiopathic pulmonary fibrosis, cystic fibrosis, and lung disease in people with HIV disease.
Healthcare providers give glutathione as a shot (by injection into the muscle) for preventing poisonous side effects of cancer treatment (chemotherapy) and for treating the inability to father a child (male infertility). Healthcare providers also give glutathione intravenously (by injection into the vein, by IV) for preventing “tired blood†(anemia) in kidney patients undergoing hemodialysis treatment, preventing kidney problems after heart bypass surgery, treating Parkinson’s disease, improving blood flow and decreasing clotting in individuals with “hardening of the arteries†(atherosclerosis), treating diabetes, and preventing toxic side effects of chemotherapy.
What is Glutathione?
|
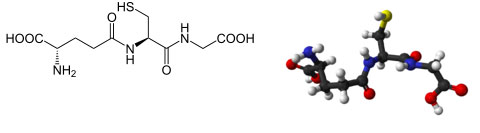 |
| (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid |
| |
Glutathione is a sulfur-containing amino acid that is an important part of the body's antioxidant defense system. Glutathione is composed of three different amino acids: cysteine, glutamic acid, and glycine. Vitamins B6 and riboflavin are critical for maintaining adequate levels of glutathione within the body.
Research suggests that a variety of minerals, including copper and selenium, have a strong influence on cellular levels of glutathione. In addition, selenium is an essential part of many different forms of glutathione that exist in the body. Because of its antioxidant properties, glutathione neutralizes damaging free radicals and peroxide molecules, and recharges oxidized vitamin C, so that the body may reuse it. Glutathione is required for a variety of metabolic processes. In addition, glutathione bolsters the structure of body proteins and assists in the transport of amino acids across cell membranes.
How is it Made?
Glutathione is not an essential nutrient, since it can be synthesized from the amino acids L-cysteine, L-glutamic acid, and glycine. The sulfhydryl (thiol) group (SH) of cysteine serves as a proton donor and is responsible for the biological activity of glutathione. Provision of this amino acid is the rate-limiting factor in glutathione synthesis by the cells, since cysteine is relatively rare in foodstuffs. Furthermore, if released as the free amino acid, cysteine is toxic and spontaneously catabolized in the gastrointestinal tract and blood plasma.
Glutathione is synthesized in two adenosine triphosphate-dependent steps:
|
 |
First, gamma-glutamylcysteine is synthesized from L-glutamate and cysteine via the enzyme gamma-glutamylcysteine synthetase (a.k.a. glutamate cysteine ligase, GCL). This reaction is the rate-limiting step in glutathione synthesis. |
|
|
 |
Second, glycine is added to the C-terminal of gamma-glutamylcysteine via the enzyme glutathione synthetase. | |
| |
|
Where is it Found?
It is found in fruits, vegetables, and meats. Limonene is found in citrus fruit peels, cherries, green foods (celery, fennel), soy products, and wheat. Several foods contain naturally occurring glutathione, including avocado, watermelon, asparagus, grapefruit, potato, acorn squash, strawberries, orange, tomato, cantaloupe, broccoli, okra, peach, zucchini, and spinach. |
| |
|
|
|
| Benefits / Uses |
 |
It is found in almost all living cells. The liver, spleen, kidneys, pancreas, and the lens and cornea, have the highest concentrations in the body. |
|
|
 |
It is a powerful antioxidant and thus neutralizes free radicals and prevents their formation. |
|
|
 |
Important role in immune function via white blood cell production and is one of the most potent anti-viral agents known. |
|
|
 |
It is one of the strongest anti-cancer agents manufactured by the body. |
|
|
 |
Glutathione is able to reduce oxidized Vitamin C and Vitamin E back to their unoxidized state. |
|
|
 |
It is used by the liver to detoxify many toxins including formaldehyde, acetaminophen, benzpyrene and many other compounds and plays a key role in Phase I and Phase II detoxification reactions. |
|
|
 |
It is an antioxidant necessary for the protection of proteins; is involved in nucleic acid synthesis and plays a role in DNA repair. |
|
|
 |
It maintains the cellular redox potential. |
|
|
 |
Glutathione levels decrease with age. It is involved in cellular differentiation and slows the aging process. |
|
|
 |
Protects the integrity of red blood cells. |
|
|
 |
Glutathione is involved in maintaining normal brain function. | |
| |
Dosage
When taken by mouth, most glutathione dosages range from 50 mg to 600 mg. However, it should be noted that research suggests that glutathione is not significantly absorbed into the body when taken by mouth (so Scientists have also developed sublingual form of Glutathione). When inhaled, a dose of 600 mg twice daily has been recommended. When given as an intramuscular injection, a dose of 600 mg every day or every other day has been suggested by some clinical studies. A few studies that have used glutathione intravenously (IV) have used doses based on body surface area (a measure calculated using your height and weight). The best way to increase Glutathione is to consume its precursor like NAC, which is highly absorbed in the body.
Possible Side effects / Precautions / Possible Interactions
Glutathione is possibly safe for most adults when taken by mouth, by inhalation, or by injection into the muscle or into the veins. But the possible side effects are not known.
Special Precautions & Warnings:
Pregnancy and breast-feeding: Not enough is known about the use of glutathione during pregnancy and breast-feeding. Stay on the safe side and avoid use.
Asthma: Do not inhale glutathione if you have asthma. It can increase some asthma symptoms.
Research Studies / References
|
 |
Merck Index, 11th Edition, 4369. |
|
|
 |
Pompella, A; Visvikis, A; Paolicchi, A; De Tata, V; Casini, AF (2003). "The changing faces of glutathione, a cellular protagonist". Biochemical Pharmacology 66 (8): 1499–503. doi:10.1016/S0006-2952(03)00504-5. PMID 14555227. |
|
|
 |
Pastore, Anna; Piemonte, Fiorella; Locatelli, Mattia; Russo, Anna Lo; Gaeta, Laura Maria; Tozzi, Giulia; Federici, Giorgio (2003). "Determination of blood total, reduced, and oxidized glutathione in pediatric subjects". Clinical Chemistry 47 (8): 1467–9. PMID 11468240. |
|
|
 |
http://www.drugs.com/pdr/immunocal-powder-sachets.html |
|
|
 |
^ Dalton, T (2000). "Knockout of the Mouse Glutamate Cysteine Ligase Catalytic Subunit (Gclc) Gene: Embryonic Lethal When Homozygous, and Proposed Model for Moderate Glutathione Deficiency When Heterozygous". Biochemical and Biophysical Research Communications 279: 324–9. doi:10.1006/bbrc.2000.3930. |
|
|
 |
Yang, Y.; Dieter, MZ; Chen, Y; Shertzer, HG; Nebert, DW; Dalton, TP (2002). "Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(-/-) knockout mouse. Novel model system for a severely compromised oxidative stress response". Journal of Biological Chemistry 277 (51): 49446–52. doi:10.1074/jbc.M209372200. PMID 12384496. |
|
|
 |
Giordano, G; Afsharinejad, Z; Guizzetti, M; Vitalone, A; Kavanagh, T; Costa, L (2007). "Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency". Toxicology and Applied Pharmacology 219 (2-3): 181–9. doi:10.1016/j.taap.2006.09.016. PMID 17084875. |
|
|
 |
McConnachie, L. A.; Mohar, I.; Hudson, F. N.; Ware, C. B.; Ladiges, W. C.; Fernandez, C.; Chatterton-Kirchmeier, S.; White, C. C. et al. (2007). "Glutamate Cysteine Ligase Modifier Subunit Deficiency and Gender as Determinants of Acetaminophen-Induced Hepatotoxicity in Mice". Toxicological Sciences 99 (2): 628–36. doi:10.1093/toxsci/kfm165. PMID 17584759. |
|
|
 |
Chen, Ying; Yang, Yi; Miller, Marian L.; Shen, Dongxiao; Shertzer, Howard G.; Stringer, Keith F.; Wang, Bin; Schneider, Scott N. et al. (2007). "Hepatocyte-specificGclcdeletion leads to rapid onset of steatosis with mitochondrial injury and liver failure". Hepatology 45 (5): 1118–28. doi:10.1002/hep.21635. PMID 17464988. |
|
|
 |
Hothorn, M.; Wachter, A; Gromes, R; Stuwe, T; Rausch, T; Scheffzek, K (2006). "Structural Basis for the Redox Control of Plant Glutamate Cysteine Ligase". Journal of Biological Chemistry 281 (37): 27557–65. doi:10.1074/jbc.M602770200. PMID 16766527. |
|
|
 |
Hicks, L. M.; Cahoon, R. E.; Bonner, E. R.; Rivard, R. S.; Sheffield, J.; Jez, J. M. (2007). "Thiol-Based Regulation of Redox-Active Glutamate-Cysteine Ligase from Arabidopsis thaliana". The Plant Cell Online 19: 2653–61. doi:10.1105/tpc.107.052597. |
|
|
 |
Wachter, Andreas; Wolf, Sebastian; Steininger, Heike; Bogs, Jochen; Rausch, Thomas (2004). "Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae". The Plant Journal 41 (1): 15–30. doi:10.1111/j.1365-313X.2004.02269.x. PMID 15610346. |
|
|
 |
Pasternak, Maciej; Lim, Benson; Wirtz, Markus; Hell, RüDiger; Cobbett, Christopher S.; Meyer, Andreas J. (2007). "Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development". The Plant Journal 53 (6): 999–1012. doi:10.1111/j.1365-313X.2007.03389.x. PMID 18088327. |
|
|
 |
Copley, Shelley D; Dhillon, Jasvinder K (2002). Genome Biology 3: research0025.1. doi:10.1186/gb-2002-3-5-research0025. |
|
|
 |
Grill D, Tausz T, De Kok LJ (2001). Significance of glutathione in plant adaptation to the environment. Springer. ISBN 1402001789. http://books.google.com/?id=aX2eJf1i67IC&pg=PA13. |
|
|
 |
Scholz RW. Graham KS. Gumpricht E. Reddy CC. Mechanism of interaction of vitamin E and glutathione in the protection against membrane lipid peroxidation. Ann NY Acad Sci 1989:570:514-7. Hughes RE. Reduction of dehydroascorbic acid by animal tissues.Nature 1964:203:1068-9. |
|
|
 |
Clementi, Emilio; Brown, Guy Charles; Feelisch, Martin; Moncada, Salvador; Bugg, S; O'Connell, MJ; Goldsbrough, PB; Cobbett, CS (1999). "Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe.". The Plant cell 11 (6): 1153–64. doi:10.2307/3870806. PMID 10368185. PMC 144235. http://jstor.org/stable/3870806. |
|
|
 |
Matsuki, Mitsuo; Watanabe, Toshihiko; Ogasawara, Ayako; Mikami, Takeshi; Matsumoto, Tatsuji (2008). "Inhibitory Mechanism of Melanin Synthesis by Glutathione". Yakugaku Zasshi 128 (8): 1203–7. doi:10.1248/yakushi.128.1203. PMID 18670186. |
|
|
 |
Noctor, Graham; Foyer, Christine H. (1998). "ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control". Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. doi:10.1146/annurev.arplant.49.1.249. PMID 15012235. |
|
|
 |
Ha, S.-B. (1999). "Phytochelatin Synthase Genes from Arabidopsis and the Yeast Schizosaccharomyces pombe". The Plant Cell Online 11: 1153–64. doi:10.1105/tpc.11.6.1153. |
|
|
 |
Parisy, Vincent; Poinssot, Benoit; Owsianowski, Lucas; Buchala, Antony; Glazebrook, Jane; Mauch, Felix (2006). "Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis". The Plant Journal 49 (1): 159–72. doi:10.1111/j.1365-313X.2006.02938.x. PMID 17144898. |
|
|
 |
Rouhier, Nicolas; Lemaire, StéPhane D.; Jacquot, Jean-Pierre (2008). "The Role of Glutathione in Photosynthetic Organisms: Emerging Functions for Glutaredoxins and Glutathionylation". Annual Review of Plant Biology 59: 143–66. doi:10.1146/annurev.arplant.59.032607.092811. PMID 18444899. | |
| | |
|